ICAN®—International Cancer Advocacy Network
ICAN® is a 501(c)(3) patient advocacy and
research advocacy organization with a small staff of professional advocates assisted by hundreds
of volunteers worldwide. With our volunteer leadership representing
17 different time zones, we literally work around the clock,
focusing on our Personalized Medicine Cancer Case Navigation
Programs, health information technology issues, legislative
initiatives, and research projects.
We aim to provide you with the best patient advocates and the most effective cancer patient advocacy possible, with the twin goals of working to extend your life with
the highest quality of life.
ICAN® advocates for you – the patient – or for the patient's spouse or family member who wants to manage the details of their loved one's cancer case with us.
We are beholden to no one – no oncology practice,
no medical center, no clinical trial, and no biotech or
pharmaceutical company.
Your case is unique and in our many years of
non-stop advocacy services, we've never seen two patient cases
remotely alike.
ICAN® empowers you to navigate rapidly-expanding
diagnostic and personalized treatment options. This information will assist you in informed discussions with
family and providers.
We never, ever, compromise patient privacy (zealous implementers of HIPAA) – We will not disclose your name or any identifying information, or exploit your case for fundraising purposes.
ICAN is very grateful to Quint Studer for recognizing our patient services—and how we view patients—in his column that reaches tens of thousands of readers: Read Quint's article here.
In the News!
A New International Research Consortium to Battle Two Rare Cancer Mutations
In April 2016, Kevin Hanlon, an entrepreneur from Syracuse, New York, was diagnosed with an EGFR exon 20 insertion-mutated lung cancer. There are two types of exon 20 insertions, the other is a HER2 insertion. Both are rare gene mutations diagnosed in two percent of non-small cell lung cancer patients. Exon 20 insertions have also been found in 24 other cancers so far. Many of the patients diagnosed with these mutations are, like Hanlon, never-smokers in excellent shape....
— Marcia K. Horn JD, ICAN President and CEO and Exon 20 Group Executive Director
Published in The Future of Health (USA Today Mediaplanet digital supplement on Lung Health). Read the article
Watch a short video clip from Ellrose Hanlon, daughter of Exon 20 Group Co-Founder of cherished memory, Kevin Hanlon
Know Your Precise Cancer Diagnosis
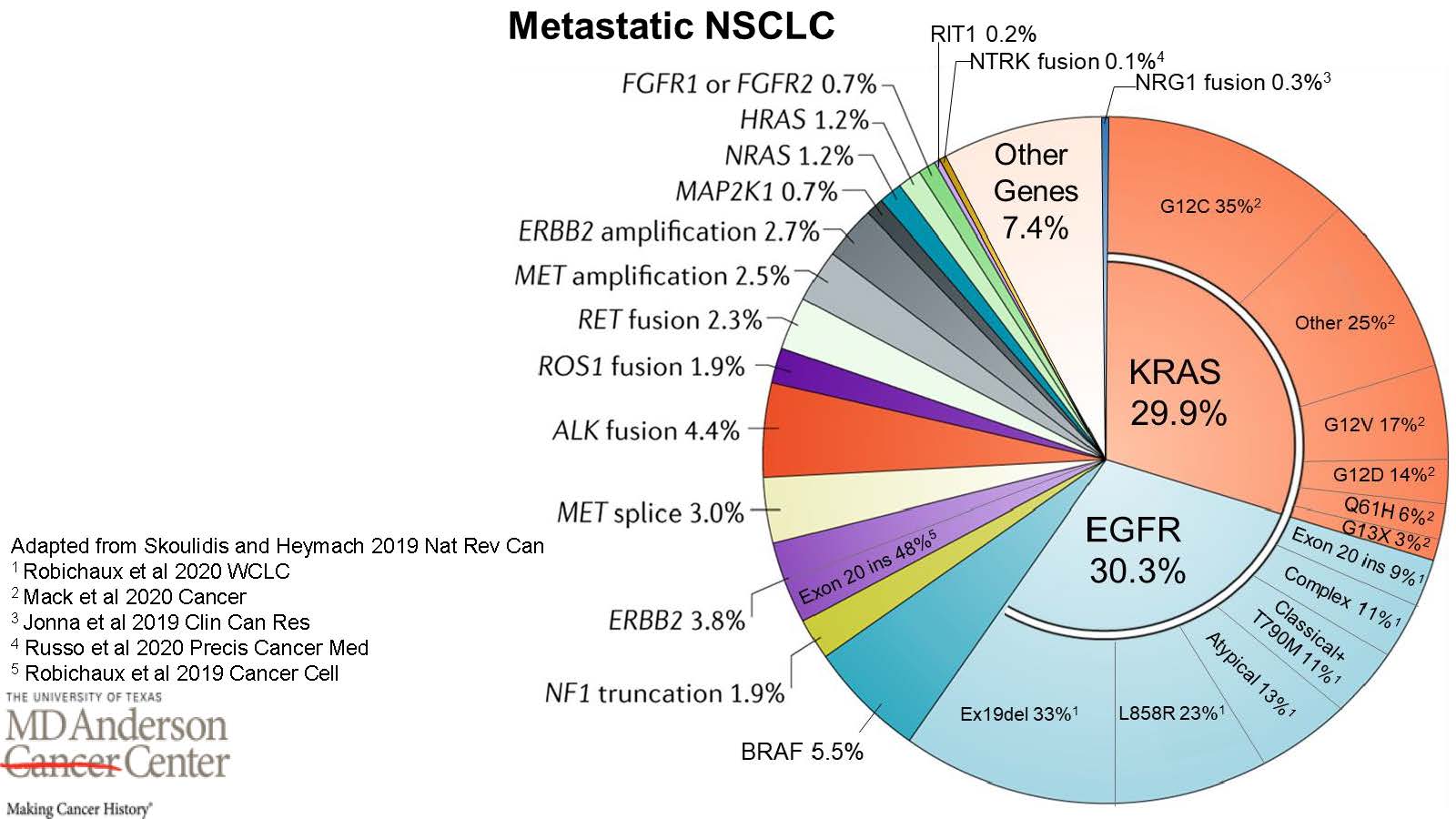
If your oncologist cannot tell you what molecular subtype of cancer you have, then you may not have a specific diagnosis yet. And your surgical or biopsy tissue might not have been adequately profiled if your physician did not order comprehensive biomarker testing. Here's a pie chart, created by the brilliant Jacqulyne ("Jackie") Robichaux, PhD, member of the John V. Heymach, MD, PhD Laboratory and Assistant Professor at The University of Texas MD Anderson Cancer Center, which reveals the molecular subtypes of Non-Small Cell Lung Cancer. If you know your particular molecular subtype, treatment decisions will likely be more targeted. If comprehensive biomarker testing does not reveal a specific molecular subtype, then that's also important information for you and your oncologist to have.
Expand Chart InfoGraphic or select (PPTX) (Text Only) (JPG)