ICAN News
Breaking News!
- Rybrevant injections may help Stage 4 lung cancer patients extend their lives—ABC 7 Eyewitness News
- FDA Grants Second Approval under the National Priority Voucher Pilot Program— FDA U.S. Food & Drug Administration
- My Diagnosis Story: EGFR Exon 20 Insertion Mutation By Mary Rokhvadze, as told to Rachel Reiff Ellis—Web MD
- The podcast link below is a fascinating discussion about an issue that concerns us all--the problems and prospects for drug development in the current political and economic environment. One of the panelists is Steve Potts, PhD, MBA, who is a venture capitalist and a noted drug developer himself. We are delighted that Steve also serves as the Chair of ICAN's Drug Development Council and advises ICAN on issues that affect drug investment, research and development, and governmental approval. The podcast lasts 42 minutes. There is also a transcript if you prefer to read the discussion.
David Hong, MD, Steve Potts, PhD, MBA, and Vivek Subiah, MD talk Pan-Tumor Approvals in a Post-IRA World —Leerlink Partners - See Exon 20 Group member Heather Quintana's interview in People magazine Mother of 2 Thought She Had a 'Very Bad Cold' That Wouldn't Go Away. Then Doctors Found 'Cancer Everywhere'—People.com | Heather Quintana Suchan Research Advocacy Program
- U.S. FDA grants accelerated approval to Boehringer's HERNEXEOS® as first orally administered targeted therapy for previously treated patients with HER2-mutant advanced NSCLC—Boehringer-Ingelheim
"The advocacy community is thrilled about the approval of zongertinib, as it is another testament to the importance of personalized options for lung cancer that allow for a much more targeted approach for a subgroup of patients who have been waiting many years for innovative treatments," said Marcia Horn, President and CEO, International Cancer Advocacy Network and Executive Director of the Exon 20 Group/HER2 Warriors. "Understanding your cancer's unique biomarkers, including HER2, through comprehensive testing is critical for all patients with NSCLC, as it can unlock targeted treatment options."
- Trump's Drug Pricing Order Could Cost Cancer Patients the Cures They Need—DC Journal, Opinion Posted to Healthcare June 20, 2025 by Andrew Spiegel, Marcia K. Horn
- As Congress Focuses on the Budget – Life Science Leaders Focus on an EPIC solution—AZBio.org
- Perspective: Life sciences in peril—Denver Gazette
- RYBREVANT® (amivantamab-vmjw) in Combination With Chemotherapy Is the First FDA Approved Therapy for First-line Treatment of Patients With Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations —Janssen Global
- HER2 Biomarker Isn’t Just About Her —Upstage Lung Cancer
- Safety, Tolerability, and Antitumor Activity of Zipalertinib Among Patients With Non–Small-Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 20 Insertions— Journal of Clinical Oncology
- Lung Cancer Considered Podcast featuring Marcia Horn discussing exon 20 issues and the FDA-approved exon 20 drugs—International Association for the Study of Lung Cancer (IASLC)
- The Importance of Molecularly Defined Patient Advocacy Groups —Lighthouse, by Celerity Biosciences
- Three questions to help those with newly diagnosed cancer:—Steve Henderson, Ph.D.'s Post
- Marcia Horn Discusses Advancements in EGFR Exon 20 Insertion+ NSCLC and the Importance of Community—AJMC (American Journal of Managed Care)
- Exon 20 Group Patient Advocate Katina Bland took part in a discussion panel featured in "If You Have Lungs You Can Get Lung Cancer: Part II" by Impact Church of East Point, Georgia. This panel starts at 43:27 into the video.
- Exon 20 Group Patient Advocate Katina Bland was interviewed in a series of videos explaining the patient journey and the importance of Next Generation Sequencing—CURE® Media Group
- Arizona Governor Doug Ducey signs legislation requiring health insurance plans to cover biomarker testing when there’s a clinical need—Office of the Governor of Arizona, news release May 5, 2022
- The Gratitude Symposium—May 2022, Hosted by Quint Studer & The Gratitude Group— A Free month-long symposium to thank healthcare workers. ICAN CEO Marcia K. Horn is part of the lineup!
- Navigating a Lung Cancer Diagnosis: 4 Key Considerations for Patients—Lisa Cruz, Patient Advocacy Lead, Takeda Oncology and Marcia K. Horn, Executive Director, Exon 20 Group at ICAN, International Cancer Advocacy Network. Published in Cure
- Opinion: Congress must turn tide in war on cancer—Sidney M. Rosen, JD, Founding Chairman of ICAN. Published in Scottsdale Progress
- Announcing the Biomarker Collaborative and NeoGenomics Strategic Partnership, benefiting the Exon 20 Group at ICAN and other Rare Mutation Groups—NeoGenomics, Inc.
- New Treatments for Non-Small Cell Lung Cancer With an EGFR Mutation: What to Know—American Society of Clinical Oncology
- Structure-based classification predicts drug response in EGFR-mutant NSCLC—Nature
"One of the most important journal articles written in this field-- Bravo to Jacqulyne Ponville Robichaux, PhD, John Heymach,MD, PhD, and members of the John V. Heymach Laboratory at The University of Texas MD Anderson Cancer Center."—Marcia K. Horn, JD - FDA Approves Mobocertinib for NSCLC with EGFR Exon 20 Insertion Mutations—Oncology Nursing News
"As a patient advocate working with EGFR Exon20 insertion+ NSCLC patients and their families every day for nearly five years, I am thrilled to witness continued progress in the fight against this devastating disease and am grateful for the patients, families, healthcare professionals and scientists across the globe who contributed to the approval of this promising targeted therapy." —Marcia Horn, executive director, Exon 20 Group at ICAN, International Cancer Advocacy Network. - Takeda's EXKIVITY™ (mobocertinib) Approved by U.S. FDA as the First Oral Therapy Specifically Designed for Patients with EGFR Exon20 Insertion+ NSCLC —Business Wire
− Approval based on Phase 1/2 trial results, which demonstrated clinically meaningful responses with a median duration of response (DoR) of approximately 1.5 years
− Next-generation sequencing (NGS) companion diagnostic test approved simultaneously to support identification of patients with EGFR Exon20 insertion mutations - Mobocertinib for Patients with EGFR Exon 20 Insertion-Positive Metastatic NSCLC with Disease Progression on Prior EGFR TKI Therapy
Marcia K. Horn, JD, Executive Director, the Exon 20 Group and CEO, ICAN, International Cancer Advocacy Network—IASLC
In reference to: OA15.01 "Mobocertinib in EGFR Exon 20 Insertion–Positive Metastatic NSCLC Patients With Disease Control on Prior EGFR TKI Therapy" presented by Dr. Alexander I. Spira | (PDF) - Phase I studies of DZD9008, an oral selective EGFR/HER2 inhibitor for patients with advanced NSCLC and EGFR Exon20 insertion mutations
Marcia K. Horn, JD, Executive Director, the Exon 20 Group and CEO, ICAN, International Cancer Advocacy Network—IASLC
In reference to: OA15.02 "Phase 1 studies of DZD9008, an oral selective EGFR/HER2 inhibitor in advanced NSCLC with EGFR Exon20 insertion mutations," presented by Dr. Pasi Jänne | (PDF)
Adopting Consensus Terms for Testing in Precision Medicine—AASCO Journal of Clinical Oncology
"Despite the well-understood benefits of biomarker and genetic testing in precision medicine, uptake remains low, particularly for patients with low socioeconomic status and minority ethnic backgrounds... A LUNGevity Foundation... assessed and recommended specific terms for communicating with patients on testing for tumor characteristics and germline mutations. Read this article
- Evaluating the Content Validity, Clarity, and Relevance of Two Patient-Reported Outcomes for Use With Adults With EGFR-Mutated NSCLC -
Marcia K. Horn, JD |
Kevin Liu, PharmD |
Susan D. Mathias, MPH |
Tracy Li, PhD |
Parthiv Mahadevia, MD |
Renee F. Pierson, MBA
Open AccessPublished:June 09, 2021DOI:https://doi.org/10.1016/j.jtocrr.2021.100198
ICAN Co-authors Letter on COVID-19 Intellectual Property Waiver
"Waiving intellectual property rights on COVID-19 vaccines initially sounds good, but is a threat to the drug discovery pipeline that cancer patients and all patients depend on."
— Marcia K. Horn, ICAN CEO and Exon 20 Group Executive Director Read the letter
First Exon 20 Drug Approved!
"Congratulations to Janssen Oncology and its principal investigators and study teams as well as to amivantamab patients and their families throughout the Exon 20 Group."
— Marcia K. Horn, ICAN CEO and Exon 20 Group Executive Director
Exon 20 Group Members Acknowledged!
Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity
"The authors wish to thank the patients, families, and caregivers involved in the clinical trial as well as the Exon 20 Group, Marcia Horn, Robert Hanlon, and Kevin Hanlon."
—See article at cell.com/cancer-cell
Highlights
A visual exploration of data presented at ASCO 2021 Virtual Meeting- Video(mp4) LARVOL VERI
Patient Advocate Comment: Amivantamab for Exon 20 by Marcia K. Horn JD— IASLC News
ICAN CEO, Marcia Horn, Quoted in Precision Oncology News on the COVID-19 Crisis: Precision Oncology Programs Adapting to Ensure Patient Safety, Care During Public Health Crisis.
- Op-ed: Inflation Reduction Act policies need fixing, not celebrating | The Center Square
- Congratulations to our wonderful Carmi Fazio, RN, MSN, ONA, Chair of ICAN's Oncology Nursing Council, and a tremendous advocate for patients and care partners : Improving Patient Understanding of Testing in Precision Medicine With Consistent, Plain Language Testing Terms | Oncology Nursing Society 46th Congress
- Distinguished ASU cancer researcher George R. Pettit retires after 55 years of service | ASU Now: Access, Excellence, Impact
- ICAN mentioned in Precision Oncology Trials Still Enroll Mostly White Patients Despite Initiatives, Calls to Diversify | Precision Oncology News (posted on the ICAN website with permission)
- Pan-Cancer Consortium Moves to Clarify and Promote Consistent Use of Common Terms for Biomarker and Germline Genetic Testing | BioSpace
- Arizona State University School of Molecular Science Salutes ICAN's Scientific Advisory Council Chairman Dr. G. R. Pettit
- New law expands access to oral cancer drugs in Brazil—by ICAN Hispanic and Ibero-American Council President, Professor Fernando Ferrer MBA
- Feds take steps to improve cancer care for Arizonans Arizona Capitol Times (Authored by Sidney M. Rosen, founding chairman of International Cancer Advocacy Network)
- Meeting Library | Using consistent terms in precision medicine to eliminate patient confusion | ASCO Meeting Library (ICAN CEO Marcia K. Horn is a co-author of this Abstract)
- Disrupting the Paradigm: Partnering With Oncogene-Focused Patient Groups to Propel Research | IASLC Lung Cancer News (ICAN's Exon 20 Group among the groups featured)
-
Is it finally their time? Two ADCs for cancer make the blockbuster list
BioWorld (ICAN CEO and ICAN patient quoted) - Vitrakvi receives first tumor-agnostic approval in EU
BioSpectrum - PA OPINION—Transforming the clinical trial to help us live our best lives
PA Consulting - Cancer Patients Want to Win, but is the Playing Field Level?
ROBERT A. NAGOURNEY, MD - DART Trial Shows Early Enrollment Success
SWOG
PROs--Patient Reported Outcomes--are a big priority for ICAN
 Please take a look at this article, co-authored by ICAN's CEO Marcia Horn along with a group of her favorite research advocates.
Please take a look at this article, co-authored by ICAN's CEO Marcia Horn along with a group of her favorite research advocates.
Including the patient voice in the development and implementation of patient‐reported outcomes in cancer clinical trials more...
ICAN Celebrates MDRT Foundation Grant – Read more...
 Stephen Rothschild (far right) presents MDRT Foundation check to ICAN Leadership.
Stephen Rothschild (far right) presents MDRT Foundation check to ICAN Leadership.
From Left: ICAN CEO Marcia Horn, Josefine and David Perry, 2018 ICAN Liberty Mutual Invitational Tournament Chair and Reception Host Cathy Dalzell, Tournament Chair Wendy Look, Tournament Chair Michael Friedman, and Reception Honorees, Debbie and Steve Rothschild. more...
TRK Fusion Webinar
ICAN, because of our deep involvement with rare cancers and our interest in integrating the patient and caregiver voice in clinical research, is proud to have sponsored a special webinar on TRK Fusion Cancers.
The webinar took place on Monday and is now available on YouTube.
ICAN in the Dominican Republic
Forging Relationships with Dominican Cancer Experts. Read the full article here.
ICAN Hispanic and Ibero-American Council President, Professor Fernando Ferrer MBA, visited Dominican Republic at the end of January and presented a series of conferences at various...
ICAN's Exon 20 Group Raises $25,000 for Broad Institute of MIT
Swing Fore Life Golf Invitational $25,000 Grant Presented to Broad Institute of MIT
Robert T. Hanlon, PhD, Exon 20 Group Co-Founder and Chair, presented a check for the $25,000 proceeds from the Swing Fore Life Golf Invitational to Heidi Greulich, PhD, Senior Group Leader in the Cancer Program at The Broad Institute of MIT and Harvard.
Together with Dr. Matthew Meyerson, Dr. Greulich is leading a group at the Broad Institute studying therapeutic approaches to treating cancers...

Scott Khan, PhD – Chairman, ICAN Biomarkers Council and Fernando Ferrer, MBA Chairman, ICAN Hispanic and Ibero-American Communities Council– at BioNJ Patient Advocacy Summit.
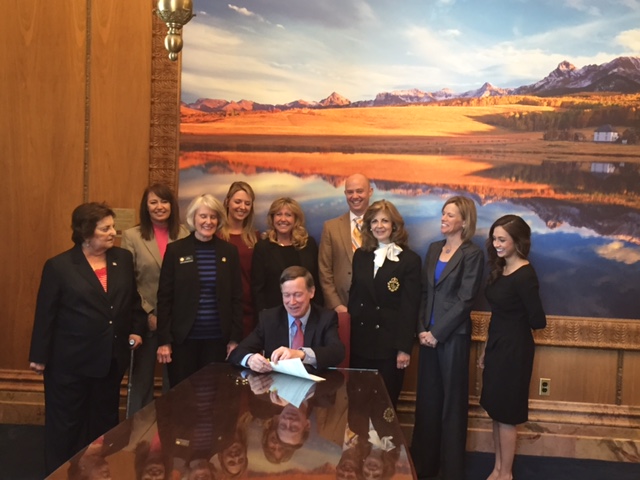
Annie Achee, member of ICAN's National Board (on the Governor's left in the black suit), is present as Colorado Governor Hickenlooper signs the biosimilars prescriber communication law. Annie and her husband, Colorado radiologist Dr. Mitch Achee, worked tirelessly to help pass this important patient safety law. Mitch, also a member of ICAN's National Board, provided compelling testimony to the Colorado legislature along with ICAN National Board members Frank Ramirez and Paul Thompson.
Federal Tax I.D.: EIN 86-0818253





